Profiles
Gyorgy Lonart, Ph.D.
Professor, Eastern Virginia Medical School, 2016 - present
Associate Professor, Eastern Virginia Medical School, 2006 - 2016
Assistant Professor, Eastern Virginia Medical School, 2011 - 2006
Assistant Professor (Research Track), University of Texas Southwestern Medical School, Dallas, 1999 - 2000
Instructor, University of Texas Southwestern Medical School, Dallas, 1996 - 1999
Instructor, University of Texas Medical Branch, Galveston, 1992 - 1996
Courses Taught
Medical courses:
Histology (Course director)
Neuroscience
Organ System Function
Graduate courses:
Concepts in Research Design (Course director)
Essentials of Physiology
Advanced Cell Biology
Cell Structure and Function
Undergraduate Degree
BS (Biology) 1984 University of Budapest
Graduate Education
PhD (Physiology) 1988 University of Budapest
Postdoctoral Education
Neurochemistry training, University of Pittsburgh, 1988 - 1990
Pharmacology training, University of Texas Medical Branch, 1990 - 1992
Research Interests
Neurological and psychiatric illnesses affect more than 50 million Americans annually, and up to one billion people around the world. These numbers underscore the importance of advancing our understanding of the human brain in health and disease.
My laboratory’s focus is synaptic transmission and mapping synaptic networks involved in fear, learning and sleep regulation. Pharmacological, physiological, and microscopic studies have made seminal contributions to our field, however the intracellular mechanisms that regulate neurotransmission remained mostly unknown until recently. Prior to becoming an EVMS faculty, I worked in the laboratory of T. C. Südhof, Nobel Prize in Medicine, 2013, to investigate the molecular mechanisms of neurotransmitter release. We have demonstrated that SNARE protein assembly-disassembly is dynamic, and that it is regulated by protein kinase C for many neurotransmitter types.
Assembly of a tripartite core complex from three SNARE protein is required for synaptic vesicle fusion. Our findings support the idea that core complex assembly represents an important point of regulation in neurotransmitter secretion and that presynaptic plasticity can operate at the step of prefusion by regulating the size of the readily releasable pool. In another series of studies, we found that Rab3 interacting molecule 1 alpha (RIM1α) is a target for protein kinase A. As an independent investigator, I extended these studies to downstream effectors of phospho-RIM1α and to another kinase, ERK, a kinase that is also important for presynaptic plasticity, likely mechanism of learning and memory.
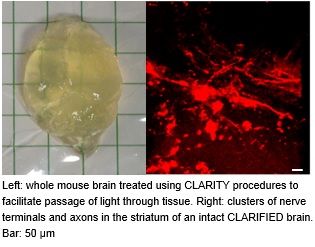
To evaluate the role of synaptic mechanisms in learning and memory we have adopted/developed behavioral techniques and collaborated with Dr. Sanford’s sleep laboratory to assess the connection of these mechanisms to sleep. We have also successfully collaborated with Dr. Britten’s lab to evaluate radiation induced changes in neurotransmission and cognitive performance. Recent additions to our technical arsenal are the CLARITY technique (see image), allowing the imaging of intact brains, and optogenetics, that facilitates functional and microscopic mapping of neuronal networks.
Current Projects
Ongoing Research Support
NIMH Lonart & Sanford (MPI) 9/6/2014 to 8/30/2016
Title: Role of amygdalar inputs to locus coeruleus in sleep regulation.
Role: Principal Investigator.
NIMH L.D. Sanford (PI) 9/26/2013 to 7/31/2018
Title: Limbic modulation of stress-induced alterations in sleep.
Role Co-Inv.
NASA R.A. Britten (PI) 2/27/2014 to 2/26/2018
Title: Changes in Neuroproteome Associated with HZE-induced Impairment of Cognition
Role: Consultant
W&M/EVMS Collaborative Grant Program 2015
PI: Sanford
Title: Neurobiological substrate of anxiety disorders
Role: Co-Inv.
Awards (selected)
Irish and Hungarian Academy of Sciences Scholarship Award
1988
Mentored Research Career Award from NIMH
1998-2002
Presentations and Scholarships
Selected Publications
Machida M, Lonart G, Sanford LD. Effects of stressor controllability on transcriptional levels of c-fos, Arc, and brain-derived neurotrophic factor in mouse amygdala and medial prefrontal cortex. Neuroreport. 2018 Jan 17;29(2):112-117. doi: 10.1097/WNR.0000000000000919. PubMed PMID: 29135807; PubMed Central PMCID: PMC5735006.
Machida M, Wellman LL, Fitzpatrick Bs ME, Hallum Bs O, Sutton Bs AM, Lonart G, Sanford LD. Effects of Optogenetic inhibition of BLA on Sleep Brief Optogenetic Inhibition of the Basolateral Amygdala in Mice Alters Effects of Stressful Experiences on Rapid Eye Movement Sleep. Sleep. 2017 Apr 1;40(4). doi: 10.1093/sleep/zsx020. PubMed PMID: 28199723
Britten RA, Jewell JS, Davis LK, Miller VD, Hadley MM, Semmes OJ, Lonart G, Dutta SM. Changes in the Hippocampal Proteome Associated with Spatial Memory Impairment after Exposure to Low (20 cGy) Doses of 1 GeV/n 56Fe Radiation. Radiat Res. 2017 Mar;187(3):287-297. doi: 10.1667/RR14067.1.
Han L, Lonart G, Sugita S.In Reply. Anesthesiology. 2016 Oct;125(4):822-3.
Liping Han, Stephen Fuqua, , Quanlin Li, Liyu Zhu, Xiaoyan Hao, Aiping Li, Sangeeta Gupta, Ravinder Sandhu, György Lonart, Shuzo Sugita, (2016) Propofol - Induced Inhibition of Catecholamine Release is Reversed by Maintaining Calcium Influx Anesthesiology, 24:878-84.
Britten RA, Davis LK, Jewell JS, Miller VD, Hadley MM, Sanford LD, Machida M, Lonart G. (2014) Exposure to Mission Relevant Doses of 1 GeV/Nucleon (56)Fe Particles Leads to Impairment of Attentional Set-Shifting Performance in Socially Mature Rats. Radiat Res. 182:292-8.
Lonart G, Parris B, Johnson AM, Miles S, Sanford LD, Singletary SJ, Britten RA.(2012) Executive function in rats is impaired by low (20 cGy) doses of 1 GeV/u (56)Fe particles. Radiat Res. 178:289-94.
Courses Taught
Medical courses:
Histology (Course director)
Neuroscience
Organ System Function
Graduate courses:
Concepts in Research Design (Course director)
Essentials of Physiology
Advanced Cell Biology
Cell Structure and Function
Undergraduate Degree
BS (Biology) 1984 University of Budapest
Graduate Education
PhD (Physiology) 1988 University of Budapest
Postdoctoral Education
Neurochemistry training, University of Pittsburgh, 1988 - 1990
Pharmacology training, University of Texas Medical Branch, 1990 - 1992
Research Interests
Neurological and psychiatric illnesses affect more than 50 million Americans annually, and up to one billion people around the world. These numbers underscore the importance of advancing our understanding of the human brain in health and disease.
My laboratory’s focus is synaptic transmission and mapping synaptic networks involved in fear, learning and sleep regulation. Pharmacological, physiological, and microscopic studies have made seminal contributions to our field, however the intracellular mechanisms that regulate neurotransmission remained mostly unknown until recently. Prior to becoming an EVMS faculty, I worked in the laboratory of T. C. Südhof, Nobel Prize in Medicine, 2013, to investigate the molecular mechanisms of neurotransmitter release. We have demonstrated that SNARE protein assembly-disassembly is dynamic, and that it is regulated by protein kinase C for many neurotransmitter types.
Assembly of a tripartite core complex from three SNARE protein is required for synaptic vesicle fusion. Our findings support the idea that core complex assembly represents an important point of regulation in neurotransmitter secretion and that presynaptic plasticity can operate at the step of prefusion by regulating the size of the readily releasable pool. In another series of studies, we found that Rab3 interacting molecule 1 alpha (RIM1α) is a target for protein kinase A. As an independent investigator, I extended these studies to downstream effectors of phospho-RIM1α and to another kinase, ERK, a kinase that is also important for presynaptic plasticity, likely mechanism of learning and memory.
To evaluate the role of synaptic mechanisms in learning and memory we have adopted/developed behavioral techniques and collaborated with Dr. Sanford’s sleep laboratory to assess the connection of these mechanisms to sleep. We have also successfully collaborated with Dr. Britten’s lab to evaluate radiation induced changes in neurotransmission and cognitive performance. Recent additions to our technical arsenal are the CLARITY technique (see image), allowing the imaging of intact brains, and optogenetics, that facilitates functional and microscopic mapping of neuronal networks.

Current Projects
Ongoing Research Support
NIMH Lonart & Sanford (MPI) 9/6/2014 to 8/30/2016
Title: Role of amygdalar inputs to locus coeruleus in sleep regulation.
Role: Principal Investigator.
NIMH L.D. Sanford (PI) 9/26/2013 to 7/31/2018
Title: Limbic modulation of stress-induced alterations in sleep.
Role Co-Inv.
NASA R.A. Britten (PI) 2/27/2014 to 2/26/2018
Title: Changes in Neuroproteome Associated with HZE-induced Impairment of Cognition
Role: Consultant
W&M/EVMS Collaborative Grant Program 2015
PI: Sanford
Title: Neurobiological substrate of anxiety disorders
Role: Co-Inv.
Awards (selected)
Irish and Hungarian Academy of Sciences Scholarship Award
1988
Mentored Research Career Award from NIMH
1998-2002
Presentations and Scholarships
Selected Publications
Machida M, Lonart G, Sanford LD. Effects of stressor controllability on transcriptional levels of c-fos, Arc, and brain-derived neurotrophic factor in mouse amygdala and medial prefrontal cortex. Neuroreport. 2018 Jan 17;29(2):112-117. doi: 10.1097/WNR.0000000000000919. PubMed PMID: 29135807; PubMed Central PMCID: PMC5735006.
Machida M, Wellman LL, Fitzpatrick Bs ME, Hallum Bs O, Sutton Bs AM, Lonart G, Sanford LD. Effects of Optogenetic inhibition of BLA on Sleep Brief Optogenetic Inhibition of the Basolateral Amygdala in Mice Alters Effects of Stressful Experiences on Rapid Eye Movement Sleep. Sleep. 2017 Apr 1;40(4). doi: 10.1093/sleep/zsx020. PubMed PMID: 28199723
Britten RA, Jewell JS, Davis LK, Miller VD, Hadley MM, Semmes OJ, Lonart G, Dutta SM. Changes in the Hippocampal Proteome Associated with Spatial Memory Impairment after Exposure to Low (20 cGy) Doses of 1 GeV/n 56Fe Radiation. Radiat Res. 2017 Mar;187(3):287-297. doi: 10.1667/RR14067.1.
Han L, Lonart G, Sugita S.In Reply. Anesthesiology. 2016 Oct;125(4):822-3.
Liping Han, Stephen Fuqua, , Quanlin Li, Liyu Zhu, Xiaoyan Hao, Aiping Li, Sangeeta Gupta, Ravinder Sandhu, György Lonart, Shuzo Sugita, (2016) Propofol - Induced Inhibition of Catecholamine Release is Reversed by Maintaining Calcium Influx Anesthesiology, 24:878-84.
Britten RA, Davis LK, Jewell JS, Miller VD, Hadley MM, Sanford LD, Machida M, Lonart G. (2014) Exposure to Mission Relevant Doses of 1 GeV/Nucleon (56)Fe Particles Leads to Impairment of Attentional Set-Shifting Performance in Socially Mature Rats. Radiat Res. 182:292-8.
Lonart G, Parris B, Johnson AM, Miles S, Sanford LD, Singletary SJ, Britten RA.(2012) Executive function in rats is impaired by low (20 cGy) doses of 1 GeV/u (56)Fe particles. Radiat Res. 178:289-94.
