Research Interests
Project I: Regulated Proteolysis in oncogenic ERBB/K-RAS-Mediated Tumorigenesis and Metastasis in human cancer
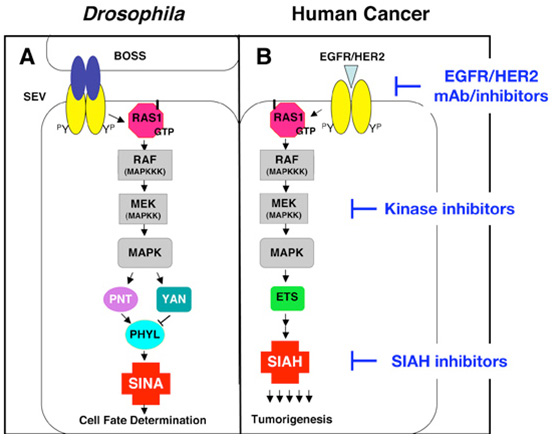
Dr. Tang's laboratory studies the RAS signal transduction pathway using multiple model organisms/systems including Drosophila, transgenic mice, human cancer cell lines and human cancer tissue specimens. As oncogenic RAS promotes the genesis of many human cancers, how best to contravene activated RAS signaling has been an intense area of investigation in the field of cancer biology for the past 30 years. Seven-In-Absentia (SINA), an E3 ubiquitin ligase, is an essential downstream component of the Drosophila RAS signal transduction pathway. The human homologue of SINA, SIAH, is a member of this evolutionarily highly conserved family of RING finger E3 ubiquitin ligases; however, the roles and regulation of SIAH-dependent proteolysis are not well understood in the context of RAS signal transduction in mammalian systems.
Dr. Tang's lab has accumulated evidence demonstrating the importance of proper SIAH function in mammalian K-RAS signaling. We show that by inhibiting the enzymatic activity of SIAH, and thus SIAH-mediated proteolysis, RAS-mediated neoplastic transformation and tumorigenesis can be effectively blocked in human cancer cells [Can Res 67(24):1798-810, 2007; JNCI 100(22):1606-29, 2008]. Furthermore, SIAH-deficient cells have reduced MAPK signaling, suggesting that SIAH might be involved in aberrant K-RAS signaling through a regulatory feedback loop mechanism. Thus, these studies provide an initial glimpse into the significance of the SIAH E3 ubiquitin ligase-regulated proteolysis in the K-RAS pathway during tumor initiation, progression and oncogenesis in human pancreatic cancer, lung cancer, invasive and metastatic breast cancer and hormonal-refractory prostate cancer.
Advancing understanding of the role of SIAH E3 ligases in K-RAS signaling and, more importantly, the potential to target SIAH as a novel new anti-K-RAS and anti-cancer target in the treatment of the most aggressive and the deadliest forms of human cancers represent exciting steps forward in the fields of K-RAS signaling, cancer biology and cancer therapy. Ultimately, we hope such SIAH-based anti-cancer therapies will lead to novel and efficacious treatments for human cancer patients, especially the ones with metastatic diseases.
Project II: Innate Immunity and Cellular Defense
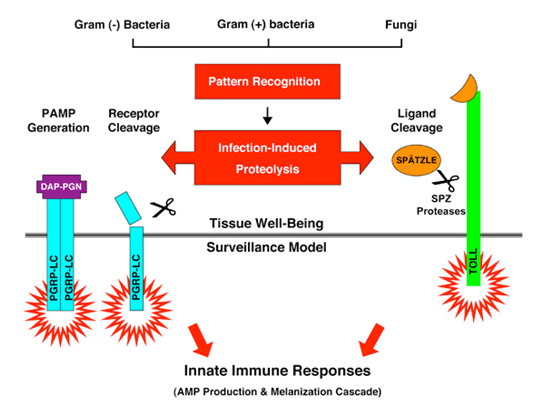
To understand how a host cell differentiates a pathogenic microbe from a nonpathogenic microorganism is a fundamental question in biology. Drosophila has an innate immune system that is similar to humans but is devoid of the complication of the adaptive immune system. We use the Drosophila as the model organism to study the molecular mechanism of how innate immunity is activated upon pathogen recognition. We found that the structural integrity of the sentinel receptors/innate sensors is modulated during infection and inflammation. We hypothesize that proteases release that is common during pathogen-host antagonism may provide an important cue for the host to distinguish a pathogenic versus a nonpathogenic microorganism. We are using transgenic fly models to demonstrate that protease release after pattern recognition provides a "tissue damage" signal that could alert host cells to the onset of endogenous tissue damage and exogenous pathogen invasion.
Project III: Genetic Screens for Anti-Cancer Drug Resistance
The development drug/chemical resistance is a recurring problem. There is an important need for us to understand the mechanisms by which drug/chemical resistance is acquired in multicellular organisms and cancers. We will carry out genetic screens in Drosophila for resistance to several key anticancer drugs that are prone to develop resistance. This effort, coupled with genomic and microarray analyses, should help to identify the alterations of key signaling pathways that could forecast and predict drug resistance development.
Research Grants Awarded
Ongoing Research Support
DOD-BCRP-BC180907 “Early Detection of Tumor Relapse in Triple Negative Breast Cancer”
Principal Investigator: Amy H. Tang, Ph.D.
Project Period: 03/01/2019 – 02/28/2022
2015-2019 CIT-CRCRF grant entitled “Development and commercialization of a new, sensitive and chemo-responsive anti-SIAH-based monoclonal antibody detection kit to examine and quantify the efficacy of chemotherapies in breast cancer patients with metastatic diseases in real time in VA” (MF14S-009-LS)
Principal Investigator: Amy H. Tang, Ph.D.
Project Period: 07/01/2015 – 12/31/2019 (NCE)
Dorothy G. Hoefer Foundation for Pancreatic Cancer Research to support the SIAH-based IHC staining to determine the prognostic value of RAS pathway biomarkers in neoadjuvant treated pancreatic cancer.
Principal Investigator: Amy H. Tang, Ph.D.
Project Period: 07/01/2016 – 06/30/2022
Dorothy G. Hoefer Comprehensive Breast Center Foundation for Breast Cancer Research to support the SIAH-based IHC staining to evaluate the efficacy of neoadjuvant therapy and individualized medicine in breast cancer.
Principal Investigator: Amy H. Tang, Ph.D.
Project Period: 01/29/2015 – 12/31/2022
EVMS Women’s and Infant Health (WIH) Grant Program entitled “Individualized Oncology at Neoadjuvant Setting for Late-Stage Metastatic Breast Cancer”
Principal Investigator: Amy H. Tang, Ph.D.
Project Period: 09/01/2017 – 11/30/2019 (NCE)
Dorothy G. Hoefer Foundation for Pancreatic Cancer Research to support the SIAH-based IHC staining to determine the prognostic value of RAS pathway biomarkers in neoadjuvant treated pancreatic cancer.
Principal Investigator: Amy H. Tang, Ph.D.
Project Period: 07/01/2016 – 06/30/2017
2014-2015 CIT-CRCRF grant entitled “Development and commercialization of a new, sensitive and chemo-responsive anti-SIAH-based monoclonal antibody detection kit to examine and quantify the efficacy of chemotherapies in breast cancer patients with metastatic diseases in real time in VA” (MF14S-009-LS)
Principal Investigator: Amy H. Tang, Ph.D.
Project Period: 07/01/2014 – 06/31/2017
Dorothy G. Hoefer Comprehensive Breast Center Foundation for Breast Cancer Research to support the SIAH-based IHC staining to evaluate the efficacy of neoadjuvant therapy and individualized medicine in breast cancer.
Principal Investigator: Amy H. Tang, Ph.D.
Project Period: 01/29/2013 – 01/28/2017
NCI “Regulated Proteolysis in Cell Migration, Tumor Growth & Metastasis in Lung Cancer" (1 R01 CA140550-01A1)
Principal Investigator: Amy H. Tang, Ph.D.
Project Period: 07/01/2010 – 06/31/2016
NIH R21 Grant application entitled “Role of the EPS Urine-Derived MicroRNA miR-888 in Prostate Cancer Progression”
Principal Investigator: Aurora Esquela Kerscher, Ph.D.
Co-investigator: Amy H. Tang, Ph.D.
Project Period: 07/01/2014 – 06/30/2016
NIH NCI R01 - 1 R01 CA140550-06
Title: "SIAH2-Dependent Proteolysis in Cell Migration, Tumor Growth and Cancer Metastasis"
Principal Investigator: Amy H. Tang, Ph.D.
NCI R01 Project Period: 07/01/2010 – 06/30/2016
AACR 2010 Pancreatic Cancer Action Network- American Association for Cancer Research (AACR) Innovative Grant (AACR-PanCan #169458)
"SIAH is a novel and effective anti-K-RAS drug target in pancreatic cancer"
Principal Investigator: Amy H. Tang, Ph.D.
Project Period: 07/01/2010 - 06/30/2013
Department of Defense "Targeting SIAH E3 Ligase Downstream of the HER2/Neu/RAS Signaling Pathway to Block Highly Invasive Human Breast Cancer Tumorigenesis and Metastasis" (DOD-Idea Award-BC095305)
Principal Investigator: Amy H. Tang, Ph.D.
Project Period: 07/01/2010 - 06/30/2013
NIGMS "Regulated Proteolysis in the RAS Signal Transduction" (R01 GM 069922)
Principal Investigator: Amy H. Tang, Ph.D.
Project Period: 05/01/2004 - 04/30/2011
NIH supplement "Regulated Proteolysis in the RAS Signal Transduction" (R01 GM 069922Z-05S1)
Principal Investigator: Amy H. Tang, Ph.D.
Project Period: 09/30/2009 - 04/30/2011
Publications
White, K. A., Swier, V. J., Cain, J.T., Kohlmeyer, J.L., Meyerholz, D. K., Tanas, M. R., Uthoff, J., Hammond, E., Li, H., Rohret, F. A., Goeken, J. A., Chan, C.H., Leidinger, M., Umesalma, S., Wallace, M. R., Dodd, R. D., Panzer, K., Tang, A. H., Darbro, B. W., Moutal, A., Cai, S., Li, W., Bellampalli, S. S., Khanna, R., Rogers, C. S., Sieren, J. C., Quelle, D. E., and J. M. Weimer (2018) A porcine model of neurofibromatosis type 1 that mimics the human disease, JCI Insight. 2018;3(12):e120402. Published on June 21, 2018, doi:10.1172/jci.insight.120402. https://doi.org/10.1172/jci.insight.120402.
Van Sciver R. E., Lee M. P., Lee C. D., Lafever A. C., Svyatova E., Kanda K., Colliver A. L., Siewertsz van Reesema L. L., Tang-Tan A. M., Zheleva V., Bwayi M. N., Bian M, Schmidt R. L., Matrisian L. M., Petersen G. M., and A. H. Tang (2018) A New Strategy to Control and Eradicate "Undruggable" Oncogenic K-RAS-Driven Pancreatic Cancer: Molecular Insights and Core Principles Learned from Developmental and Evolutionary Biology. Cancers (Basel). doi: 10.3390/cancers10050142. Review. PMID: 29757973. Cancers2018, 10(5), 142; https://doi.org/10.3390/cancers10050142
Pepper, I., Van Sciver, R. E. and A. H. Tang (2017) Phylogenetic Analysis of the Evolutionarily Highly Conserved Family of SINA/SIAH E3 Ligases in Metazoa, BMC Evol Biol. 2017 Aug 7;17(1):182. doi: 10.1186/s12862-017-1024-x, PMID: 28784114,
https://www.ncbi.nlm.nih.gov/pubmed/28784114
Xin Jin, Yunqian Pan, Liguo Wang, Tao Ma, Lizhi Zhang, Amy H. Tang, Daniel D. Billadeau, Heshui Wu and Haojie Huang (2017) Fructose-1,6-bisphosphatase inhibits ERK activation and gemcitabine resistance in pancreatic cancer by blocking IQGAP1-MAPK interaction, Cancer Res. 2017 Jul 18, pii: canres.3143.2016. DOI: 10.1158/0008-5472.CAN-16-3143. PMID: 28720574, http://cancerres.aacrjournals.org/content/canres/early/2017/08/02/0008-5472.CAN-16-3143.full.pdf
*Siewertsz van Reesema, L. L., *Lee, M. P. Zheleva, V., Winston, J. S., O’Connor, C. F., Perry, R. R., Hoefer, R. A. and A. H. Tang (2016) RAS pathway biomarkers for breast cancer prognosis, Clinical Laboratory International (CLI), November 4 issue, pp18-23, Women’s cancer biomarkers, 2016.
http://www.clinlabint.com/detail/clinical-laboratory/ras-pathway-biomarkers-for-breast-cancer-prognosis/
Siewertsz van Reesema, L. L., Zheleva, V., Winston, J. S., Jansen, R. J., O’Connor, C. F., Isbell, A. J., Bian, M., Qin, R., Bassett, P. T., Hinson, V. J., Dorsch, K. A., Kirby, B. W., Van Sciver, R. E., Tang-Tan, A.M., Harden, E. A., Chang, D. Z., Allen, C. A., Perry, R. R., Hoefer, R. A. and A. H. Tang. "SIAH and EGFR, two RAS pathway biomarkers, are prognostic in locally advanced and metastatic breast cancer", EBioMedicine, Online publication August 14, 2016. DOI information: 10.1016/j.ebiom.2016.08.014, NIHMS 812981, PubMed Central PMCID http://www.sciencedirect.com/science/article/pii/S2352396416303632
EVMS Press Release on August 23, 2016
http://www.evms.edu/pulse/archive/evmssentaracollaborativestudymaybenefitbreastcancerpatients.php
Susan G. Komen Friday News Flash – Komen News – on August 26, 2016
Van Sciver, R. E., Njogu, M. M., Isbell, A. J., Odanga, J. J., Bian, M., Svyatova, E., Siewertsz van Reesema, L. L., Zheleva, V., Eisner, J. L., Bruflat, J. K., Schmidt, R. L., Tang-Tan, A. M., and A. H. Tang (2016) Chapter 12 – Blocking SIAH Proteolysis, an Important K-RAS Vulnerability, to Control and Eradicate K-RAS-Driven Metastatic Cancer. Conquering RAS: From Biology to Cancer Therapy, pages 213-232.
DOI: 10.1016/B978-0-12-803505-4.00012-6.(Elsevier Academic Press).
Elsevier Inc: http://www.sciencedirect.com/science/article/pii/B9780128035054000126
R. Qin, T. C. Smyrk, N. R. Reed, R. L. Schmidt, T. Schnelldorfer, S. T. Chari, G. M. Petersen and A. H. Tang (2015) Combining clinicopathological predictors and molecular biomarkers in the oncogenic K-RAS/Ki67/HIF-1α pathway to predict survival in resectable pancreatic cancer. British Journal of Cancer, 112: 514-522. (3 February 2015) doi:10.1038/bjc.2014.659
http://www.nature.com/bjc/journal/v112/n3/full/bjc2014659a.html
http://www.ncbi.nlm.nih.gov/pubmed/25584484
Burket, J. A., Benson, A.D., Tang, A. H. and S. I. Deutsch (2015) NMDA Receptor Activation Regulates Sociability by its Effect on mTOR Signaling Activity, Progress in Neuro-Psychopharmacology & Biological Psychiatry, 60: 60-65.
http://www.sciencedirect.com/science/article/pii/S0278584615000421
Vasilena Zheleva, Minglei Bian, Xiaofei Gao, Justin Odanga, Zena Urban, Monicah Njogu, Bruce Knudsen, Richard A. Hoefer, Roger R. Perry, Amy H. Tang (2014)
Inhibition of Established Pancreatic and Triple Negative Breast Tumor Growth by Blocking the Most Downstream Signaling Module, SIAH, in the Oncogenic ERBB/K-RAS Signaling Pathway
Journal of the American College of Surgeons 09/2014; 219(3):S136-S137. DOI:10.1016/j.jamcollsurg.2014.07.327, 4.45 Impact Factor
http://www.journalacs.org/article/S1072-7515%2814%2900863-1/abstract
Deutsch, S. I., Tang, A. H., Burket, J. A., Benson A. D. (2014).
NMDA Receptors on the Surface of Cancer Cells: Target for Chemotherapy?
Biomed Pharmacotherapy 68 (4): 493-496. PMID: 24751001
http://www.ncbi.nlm.nih.gov/pubmed/24751001
Burket, J. A., Benson, A.D., Tang, A. H. and S. I. Deutsch (2014).
Rapamycin Improves Sociability in the BTBR T+ Itpr3tf/J Mouse Model of Autism Spectrum Disorders.
Brain Research Bulletin. 100: 70-75. PMID: 24295733
https://www.sciencedirect.com/science/article/abs/pii/S036192301300186X
Tang, A. H. (2013).
Stopping metastasis in its tracks.
International Innovation (North America -November Issue), page 42-44. ISSN 2051-8528. PMID: 23685206
www.international-innovation-northamerica.com
Burket, J. A., Benson, A.D., Tang, A. H. and S. I. Deutsch (2013).
D-Cycloserine Improves Sociability in the BTBR T+ Itpr3tf/J Mouse Model of Autism Spectrum Disorders with Altered Ras/Raf/ERK1/2 Signaling.
Brain Research Bulletin. 96: 62-70; PMID: 23685206
http://www.sciencedirect.com/science/article/pii/S0361923013000786
Tang, A. H. (2011)
Are you my friends or are you my enemies?
Self/Nonself, Immune Recognition and Signaling. 2:3, 142-146(Volume 2 Issue 3, June - December 2011).
http://www.landesbioscience.com/journals/selfnonself/02-TangSNS2-3.pdf
Schmidt, R. L., Rinaldo, F. M., Hesse, S. E., Hamada, M., Ortiz, Z., Beleford, D. T., Page-McCaw, A., Platt, J. L. and A. H. Tang. (2011)
Protease-Dependent Activation of the Drosophila IMD Pathway in Response to Gram-Negative Bacterial Infection.
Self/Nonself, Immune Recognition and Signaling 2, 34, 17882.
http://www.landesbioscience.com/journals/selfnonself/01-SchmidtSNS2-3.pdf
Podratz, J. L., Staff, N. P., Froemel, D., Wallner, A., Wabnig, F., Bieber, A. J., Tang, A. H. and A. J. Windebank. (2011)
Drosophila melanogaster: A new model to study Cisplatin-induced neurotoxicity
Neurobiology of Diseases 43, 330-337.
http://www.sciencedirect.com/science/article/pii/S0969996111001173
Behling KC, A. H. Tang, Freydin B, Chervoneva I, Kadakia S, Schwartz GF, Rui H, Witkiewicz AK. (2010)
Increased SIAH expression predicts DCIS progression to invasive carcinoma.
Breast Cancer Research and Treatment, November 19 issue, pp1254-1258.
https://www.ncbi.nlm.nih.gov/pubmed/21088888
Ahmed, A. U., Schmidt, R. L., Park, C. H., Reed, N. R., Hesse, S. E. Thomas, C. F., Molina, J. R., Deschamps, C., Aubry, M. C. and A. H. Tang. (2008)
Effect of Disrupting Seven In Absentia Homolog 2 Function on Lung Cancer Cell Growth
Journal National Cancer Institute 100, 1606-1629.
http://jnci.oxfordjournals.org/cgi/content/full/100/22/1606.
Schmidt, R. L., Park, C. H. Ahmed, A. U., Gundelach, J.H., Reed, N. R., Cheng, S., Knudsen, B. E. and A. H. Tang. (2007)
Inhibition of RAS-Mediated Tumorigenesis by Targeting the downstream E3 Ubiquitin Ligase, SIAH.
Cancer Research, 67, 11798-11810.
http://cancerres.aacrjournals.org/cgi/content/full/67/24/11798
Schmidt, R. L., Trejo, T. R., Plummer, T. B. Platt, J. L. and A. H. Tang. (2008)
The Infection-Induced Proteolysis of PGRP-LC Controls the IMD Activation and Melanization Cascades in Drosophila.
FASEB J. 22, 918-929.
http://www.fasebj.org/cgi/content/full/22/3/918
Schmidt, R. L., Rinaldo, F. M., Hesse, S. E., Hamada, M., Ortiz, Z., Beleford, D. T., Page-McCaw, A., Platt, J. L. and A. H. Tang. (2009).
Protease-Dependent Activation of the Drosophila IMD Pathway in Response to Gram-Negative Bacterial Infection.
Innate Immunity 1, 1-15.
Tang, A. H., G. J. Brunn, M. Cascalho and J. L. Platt. (2007)
Endogenous pathway to SIRS, sepsis and related conditions.
Journal of Leukocyte Biology 82, 282-285.
http://onlinelibrary.wiley.com/doi/10.1189/jlb.1206752/full
Tang, A. H. and J. L. Platt. (2007)
Accommodation of grafts: implications for health and disease.
Human Immunology 68, 645-651.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2703470/
Reed, N. R., Schmidt, R. L., Smyrk, T. C., Qin, R. Chari, S. T., Sarr, M. G., Grande, J. P., Petersen, G. M and A. H. Tang.
Predicting Pancreatic Cancer Patient Survival by Combining Clinical and Biomarkers and Tissue Microarrays.
G. B. Johnson, G. J. Brunn, A. H. Tang, J. L. Platt (2003).
Evolutionary Clues to the Functions of the Toll-like Family as Surveillance Receptors.
Trends in Immunology 24, 19-24.
http://www.cell.com/trends/immunology/abstract/S1471-4906(02)00014-5
Tang, A. H., T. P. Neufeld, G. M. Rubin and H. -Arno J. Muller (2001).
Transcriptional Regulation of Cytoskeletal Functions and Segmentation by a Novel Maternal Pair-Rule Gene,
lilliputian. Development 128, 801-813.
http://dev.biologists.org/content/128/5/801.full.pdf
T. P. Neufeld, A. H. Tang and G. M. Rubin (1998).
A genetic screen to identify components of the sina signaling pathway in Drosophila eye development.
Genetics 148, 277-286.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1459784/
Tang, A. H., T. P. Neufeld, E. Kwan and G. M. Rubin (1997).
PHYL acts to down-regulate TTK88, a transcriptional repressor of neuronal cell fates, by a SINA-dependent mechanism.
Cell 90, 459-467.
http://www.cell.com/abstract/S0092-8674(00)80506-1
G. M. Rubin, H. C. Chang, F. Karim, T. Laverty, N. R. Michaud, D. K. Morrison, I. Rebay, A. H. Tang, M. Therrien and D. A. Wassarman (1997).
Signal transduction downstream of RAS in Drosophila.
Cold Spring Habor Symp. on Quant. Biol. Volume LXII. 347-352.
http://symposium.cshlp.org/content/62/347
Tang, A. H. and C.-P. D. Tu. (1995).
Pentobarbital-induced changes in Drosophila glutathione S-transferase D21 mRNA stability: gstD21 mRNA stability.
J Biol. Chem. 270, 13819-13825.
http://www.jbc.org/cgi/content/full/270/23/13819
Tang, A. H. and C.-P. D. Tu. (1994)
Biochemical characterization of Drosophila glutathione S-transferase D1 and D21: Drosophila DDT dehydrochlorinase
J. Biol. Chem. 269, 27876-27884.
http://www.jbc.org/cgi/reprint/269/45/27876
Z.-H. Zhang, H.-Z. Liu, A.H. Tang and Y.-D. You (1988)
The Effect of hemoglobin on the fluidity of human erythrocyte membrane.
Acta Biophysics Sinica 4, 129-133.




 Minglei Bian, PhD
Minglei Bian, PhD Yang Liao, PhD
Yang Liao, PhD Atique U. Ahmed, PhD
Atique U. Ahmed, PhD Cheol Hong Park, PhD
Cheol Hong Park, PhD Yajun Cao, PhD
Yajun Cao, PhD YingYing Liu, PhD
YingYing Liu, PhD Xiaofei Gao, PhD
Xiaofei Gao, PhD Nanette R. Reed, MD
Nanette R. Reed, MD Vassilena Zheleva, MD
Vassilena Zheleva, MD Kevin F. Kanda, MS
Kevin F. Kanda, MS Rebecca L. Schmidt, PhD
Rebecca L. Schmidt, PhD Monicah M. Njogu
Monicah M. Njogu Robby Van Sciver
Robby Van Sciver Elizaveta Svyatova
Elizaveta Svyatova Justin J. Odanga
Justin J. Odanga Andrew J. Isbell
Andrew J. Isbell Xavier-Lewis Palmer
Xavier-Lewis Palmer Zena Urban
Zena Urban Narotham Badrish
Narotham Badrish Alex C. LaFever
Alex C. LaFever Amber L. Collier
Amber L. Collier Shipra Maheshwari
Shipra Maheshwari Michael P. Lee
Michael P. Lee Dasom Caroline Lee
Dasom Caroline Lee Rahim M. Dhanani
Rahim M. Dhanani Ian Pepper, BS
Ian Pepper, BS Drake Bishop
Drake Bishop Lauren Siewertsz van Reesema, BS
Lauren Siewertsz van Reesema, BS Ting Chen, MS
Ting Chen, MS Kristin Sica
Kristin Sica John A. Crooks
John A. Crooks Michael Cameron Hayes
Michael Cameron Hayes Jamie Eisner
Jamie Eisner Bridget N. Montgomery
Bridget N. Montgomery Oscar A. Gonzales
Oscar A. Gonzales Chris Smith
Chris Smith Mahelet N. Mamo
Mahelet N. Mamo Abigail C. Cole
Abigail C. Cole Ricky D. Moscoso
Ricky D. Moscoso Stephen Tang
Stephen Tang Angela Tang-Tan
Angela Tang-Tan Domonique White
Domonique White Garrett Rushing
Garrett Rushing Chris Rowley
Chris Rowley John Edward Fernan
John Edward Fernan Russell Wilson
Russell Wilson Matthew Ambler
Matthew Ambler