Research sheds new light on how the heart works
Two EVMS scientists continue to advance medicine’s understanding of how the heart works.
In their latest research, collaborators Vitold Galkin, PhD, Associate Professor of Physiological Sciences, and Howard White, PhD, Professor of Physiological Sciences, report on their discovery of how key proteins interact to make the heart contract. Their work, “N-terminal domains of cardiac myosin binding protein-C cooperatively activate the thin filament,” is published in the journal Structure.
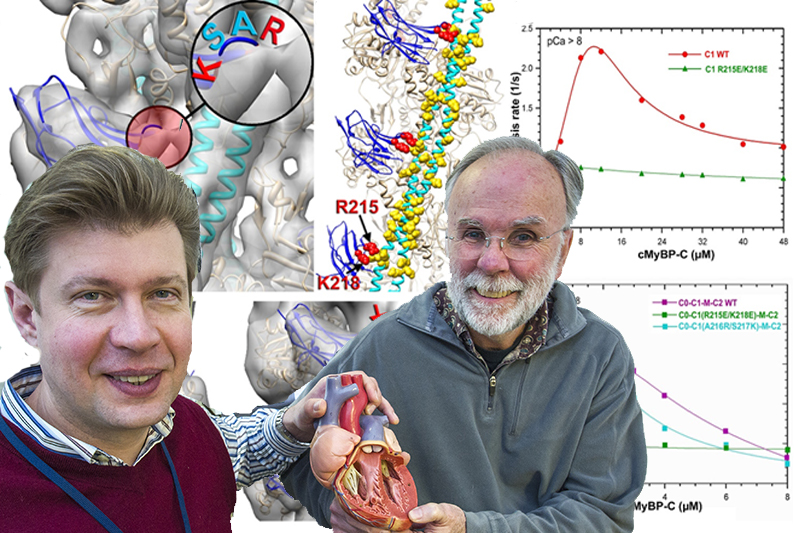
Our heart beats — or contracts — thanks to the force generated by the interaction between thick myosin and thin actin filaments, says Dr. Galkin. While much is known regarding the structure and function of myosin, relatively little is known regarding its partner protein, cardiac myosin binding protein-C (cMyBP-C).
“cMyBP-C is an essential regulatory protein in the heart that is necessary for normal cardiac contraction and is required for increased contractility — or a more rapid heartbeat — in response to fight-or-flight stimuli,” he says. “The importance of cMyBP-C to cardiac function is further highlighted because cMyBP-C is altered during heart failure, and mutations in the gene encoding cMyBP-C are the most common cause of hypertrophic cardiomyopathy (HCM), the leading cause of sudden cardiac death in young people.”
In their research, Drs. Galkin and White demonstrate that cMyBP-C regulates the interaction between the thick and thin filaments by means of a short, positively charged loop located in its C1 N-terminal domain. The researchers have demonstrated that the modulation of the charge of this loop either inhibits or enhances the activation effect of the cMyBP-C on the cardiac thin filament.
Their work has helped identify a unique target for drugs designed to control the heartbeat, which may open new avenues in treating two common forms of heart disease: hypertrophic and ischemic cardiomyopathies.
You can find the paper online at cell.com.